
Comprehensive HPTLC fingerprinting analysis of sage leaves: simultaneous identification of flavonoids and phenylpropanoids, and quantitative determination of rosmarinic acid.
By Dr. Kateryna Khokhlova, President of the NIS Chapter
In 2020, our research group published an article in Ukrainian showing the application of a new HPTLC method for quality evaluation of sage, based on the "comprehensive HPTLC fingerprinting" concept [1]. This blog summarized that publication for the English speaking HPTLC community.
Multiple herbal products containing sage leaves are present in the Ukrainian market, including tinctures, extracts, teas, and multicomponent herbal drugs. While the Ukrainian Pharmacopoeia proposes a TLC identification of essential oils and a determination of essential oils content by vapor distillation, evaluation of other groups of bioactive substances may be more suitable for identifying that herbal drug. For example, the Ukrainian sage products are usually prepared with 40-70% alcohol, which will contain more polar compounds, such as flavonoids, and less of the lower polar compounds like essential oils. Additionally, the marker tujon, used in the identification of essential oils of sage, is unstable in solution.
To explore the full potential of HPTLC methods and simplify the quality control process, we proposed a single HPTLC method for a comprehensive HPTLC fingerprint analysis of sage leaf (Salvia officinalis L.). That HPTLC method includes identification of sage leaf and quantitative determination of rosmarinic acid, a marker for this herbal drug.
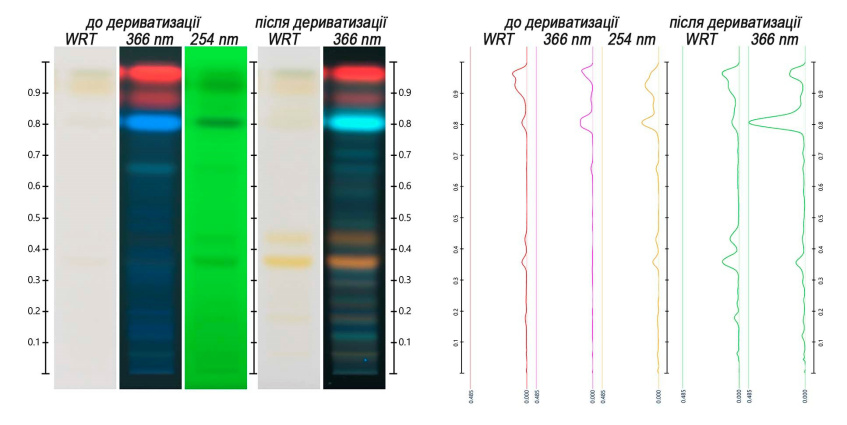
The specific flavonoids and phenylpropanoids profiles were useful for identification of sage leaf. They show several zones before and after derivatization (Figure 1) characteristic of the species, which can help distinguishing sage leaf from other herbal drugs.
To determine the potency of sage leaf, the marker rosmarinic acid was chosen. Its quantification was performed before derivatization in UV 366 nm. The quantity of that substance in sage leaves was between 5.8-15.9 mg per g of herbal drug (scanning densitometry) and 6,0-16,3 mg per g of herbal drug (image evalution). Based on this result we set the minimum content of rosmarinic acid in sage leaves at 0.4%. It means that good quality sage leaf should not contain less than 0.4% of that substance.
The quantitative determination is also useful to verify presence of other sage species because S. aethiopis and S. nemerosa have a lower content of rosmarinic acid, and thus will fail the quantitative test.
The presented approach offers a powerful alternative to the current pharmacopoeial method for sage, allowing simultaneous identification and quantitative determination at the same plate. For those users who wish to replicate that HPTLC method in their labs, we are providing the parameters below:
|
Sample preparation for identification |
100 mg/ml of powdered sage leaves in methanol, shaken for 10 min and then centrifuged. Use the supernatant as test solution. |
|
Sample preparation for quantitative determination |
1 part of the test solution is diluted with 9 parts of methanol, to produce a second test solution at 10 mg/ml. |
|
Standard preparation (ID, SST) |
0,4 mg/ml of rosmarinic acid in methanol and 0,5 mg/ml of luteolin in methanol |
|
Standard preparation (quantitative determination |
0,1 mg/ml of rosmarinic acid in methanol. This concentration is equivalent to 0.4% of rosmarinic acid in the herbal drug, on a dried basis. |
|
Stationary phase (Type, size) |
HPTLC Si 60 F254 (Merck). |
| Application volume (ID, MCT) |
2 µl of reference solutions/ calibration solutions and 4 µl of test solutions |
|
Mobile phase |
Ethyl acetate, formic acid anhydrous, water 8:1:1 (v/v/v) |
|
Saturation |
20 minutes (with saturation pad) |
|
Humidity control |
10 minutes with MgCl2 (33% relative humidity) |
|
Developing distance |
62 mm (70 mm from the lower edge) |
|
Derivatization (ID) |
Name: NP/PEG Preparation: NP: 5 mg/ml of 2-aminoethyl diphenylborinate in ethyl acetate. PEG: 50 mg/ml of polyethylene glycol 400 in dichloromethane Use: pre-heat the plate at 100°C for 3 minutes. Dip the warm plate into NP and then in PEG, using the following parameters: dipping speed: 5, dipping time: 0. |
|
Detection |
Record images in white light, UV 254 nm, UV 366 nm before derivatization; and white light, UV 366 nm after derivatization |
|
Detection (quantitative determination) |
Convert images in UV 366 nm before derivatization into peak profiles from images (Figure 1) |
|
Densitometry (quantitative determination) |
UV 366 nm before derivatization with TLC Scanner, absorbance mode, slit dimension 5,0 x 0,2 mm, scanning speed 20 mm/s, spectra recording from 190 to 450 mm. |
Comments
Kateryna Khokhlova
- February 22, 2021 at 10:51Dr.
Please, share your experience from the practice, have you faced issues with the analysis of Sage of your region by essential oils profiles? Was tujon - the specific marker that is used in Europoean Pharmacopoeia for discrimination of related Sage species, present in the Salvia officinalis leaves/herbal products? How many samples were analyzed? Which sample preparation was used? How stable tujon was? It would be interesting to know...
Leave a comment