HPTLC as a quality control method suitable for laboratories with different budgets
By Débora Frommenwiler and Maged Sharaf
In West African countries, where very little investment is available for research, particularly in herbal medicines, having low-cost technologies is an excellent alternative to ensure products' quality. HPTLC proves to be suitable for this purpose.
In low-income countries, e.g., those in West Africa, access to health care and pharmaceutical products is costly and limited. Therefore, various people use traditional herbal medicines for treating health conditions [1]. Many of the herbal medicines are produced and consumed locally.
For ensuring herbal medicines' quality and safety, a set of tests is usually applied to verify the identity, presence of contaminants, and concentration of ingredients per unit dose. Different technologies and instruments are used and it is estimated that 10-20 tests are required, which can have an associated high cost. This may prove prohibitive where financial res.
In low-income African countries, where the investments in research centers, particularly in rural areas, are limited, quality evaluations are usually not performed. Those sites often lack qualified personnel, analytical instruments, and laboratory material.
Mr. Kevin Clark presents an example of that in his master thesis [2], in which he studied the quality control of Diabefla®, an herbal preparation from Phytofla Labs used in Burkina Faso to treat diabetes. According to his work, only pesticide and microbiological investigations are carried out in traditional herbal medicines registered in Burkina Faso. However, the material's identity, chemical composition, or absence of adulteration remains unknown.
In this context, the choice of an analytical technique that can provide information about quality and be adapted to low-income African countries' specific economic needs is of high importance. The technique needs to have low solvent consumption due to safe disposal problems; have robust instruments due to difficulties in maintenance and delivery of spare parts; have low-cost and easy-to-use instruments for the producers in the rural area; be simple, efficient, and reliable.
Having these conditions in mind, Mr. Clark found his target in HPTLC being a high-end analytical technique that is adaptable to low-cost circumstances and providing reliable results.
First, he developed an HPTLC method to identify Sclerocarya birrea leaf (the herbal drug used for treating diabetes). His goal was to evaluate the chemical composition of this herbal drug. He found out that it contains a particular pattern composed of substances belonging to the class of flavonoids. The method's capability to detect adulteration with confounding material (e.g., different parts of S. birrea and other species) was investigated. The method could detect the presence of material, other than S. birrea leaf, due to its different fingerprint pattern. He also proved that with this HPTLC method, quantifying total flavonoids was possible using the image evaluation (no need for an additional instruments).
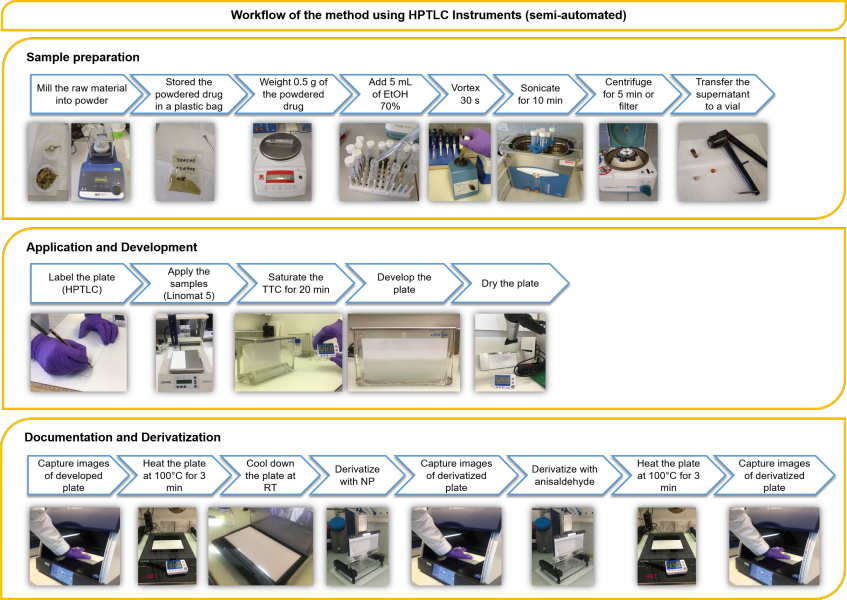
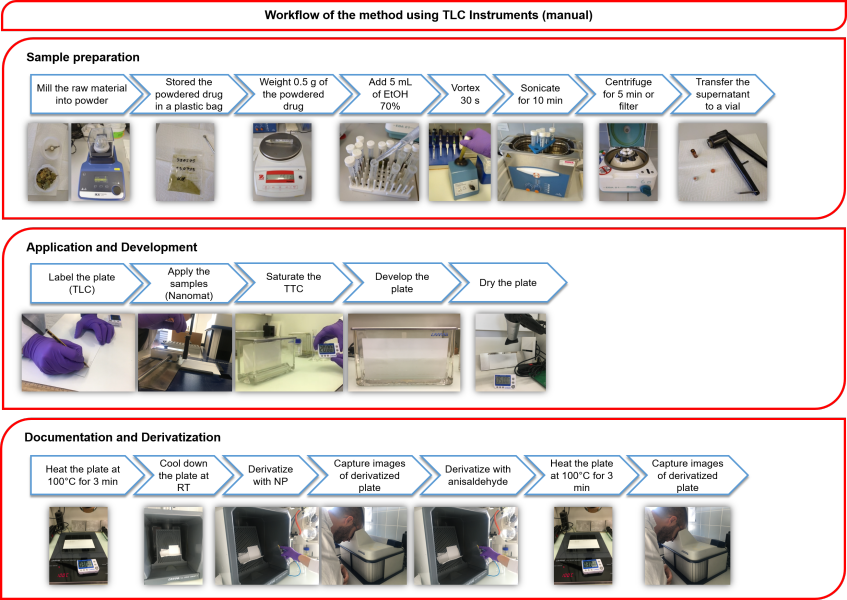
Furthermore, Mr. Clark was able to obtain similar results using instrumental and manual HPTLC. These two scenarios addressed situations when one or more pieces of the HPTLC instrument (e.g., automatic developing chamber) is lacking and for laboratories with no equipment. Three workflows representing the high to low end scenarios are shown in Figures 1-3. They were copied from his master thesis. Results obtained with the low-cost and manual setups could be compared to those obtained with full HPTLC instrumentation. For example, after a one-hour analysis performed manually, the analyst can visually compare the image observed in the UV cabinet with a reference picture which was obtained with full instrumentation and determine whether or not the fingerprint looks similar. This work proves that HPTLC is a technology suitable for quality control of herbal medicines and is accessible to laboratories with different budgets. Besides the methodology transfer, Mr. Clark also trained lab technicians and pharmacists in various research centers of Senegal and Burkina Faso (Figure 4 A&B).
[1] Actions Tenga. Centre de sante et de promotion sociale de Roumtenga. Actions Tenga; 2019.[2] Kevin Clark. Quality control of Sclerocarya birrea based preparations used in West Africa for Diabetes Mellitus: Diabefla® (Master thesis). Presented to the Faculté des sciences de L’Université de Genève in 2019. Supervisor: Dr. P. Christen

Comments
Leave a comment