
Why using HPTLC as screening tool for antioxidant activity?
By Tomislav Sostaric and Débora Frommenwiler
Among the various fields of application of the HPTLC technique, that of testing for antioxidant activity is an outstanding one. This is particularly because the HPTLC-DPPH method is fast, relatively sensitive and allows identifying individual zones with activity (effect directed analysis).
Several research groups have applied HPTLC combined with antioxidant derivatization to evaluate the antioxidant capacity of natural products. In a recent publication in the Journal of Planar Chromatography, a team at the School of Allied Health in Australia applied this technique to the analysis of Australian honey [1].
Why evaluating antioxidant capacity?
An excess of free radicals (e.g., reactive oxygen species, an extremely reactive molecule) produced by an imbalance of biological systems can damage cells causing different pathologies and aging. An increasing number of scientific studies claims that the administration of exogenous antioxidant substances contained in food, or food supplements from natural sources might help the biological system to deactivate free radicals, and consequently avoid several pathologies [2]. The evaluation of the antioxidant capacity is important for identifying natural products that can fight problems caused by free radicals.
How is the antioxidant capacity measured?
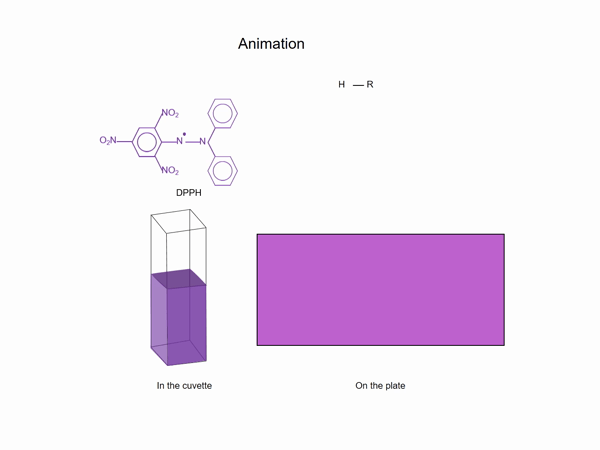
According to Alam et. al. [3], there are at least 19 in vitro and 10 in vivo methods for evaluating the antioxidant capacity. The method most frequently used for in vitro evaluation is using DPPH reagent (2,2-diphenyl-1-picrylhydrazyl or α,αdiphenyl-β-picrylhydrazyl), mainly because of its low cost, ease of reaction visualization and remarkable stability. When DPPH, an intense violet solution prepared in alcohol, is in contact with radical scavenger (antioxidant) substances, the reduced form DPPH-H is produced, changing the color of the solution from violet to yellow (GIF). Thus, the higher the antioxidant activity, the stronger the discoloration of the reagent. A similar effect is observed on the HPTLC plates, where the zones with antioxidant capacity decolorize the reagent.
While in cuvette, the antioxidant capacity is estimated as a sum of the contribution of all substances to the activity, in the HPTLC antioxidant test, the activity of individual substances is measured. The degree of discoloration of a zone/solution is compared to that obtained with the internal standard, considered as 100% scavenger.
Why testing the antioxidant capacity of honey?
As mentioned by Islam et al. [1], honey is known for its antimicrobial, antioxidant and anti-inflammatory effects. The antioxidant activity of honey is of growing interest although to date, only a few studies have investigated the specific constituents that are responsible for this effect and only limited comparative analyses have been carried out.
What was the result of the study?
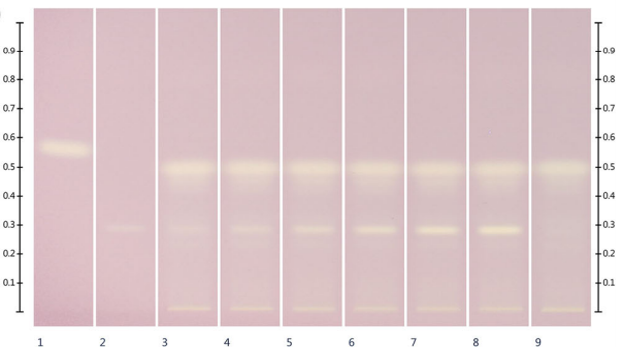
Islam et al. [1] developed and validated an HPTLC method for visualization of the compounds contributing to the antioxidant activity of honey and quantified their individual antioxidant capacity. This method was applied to a Manuka honey (produced from the nectar of the m?nuka tree) and a generic honey, available from a major supermarket chain (probably a multifloral table honey). Both honeys have 3-4 zones with antioxidant capacities (pale yellow zones such as those seen in Figure). However, Manuka honey has more than 5-fold the antioxidant capacity than the generic honey.
Comments
Leave a comment